Hydration requirements with emetogenic chemotherapy: granisetron extended-release subcutaneous versus palonosetron
Abstract
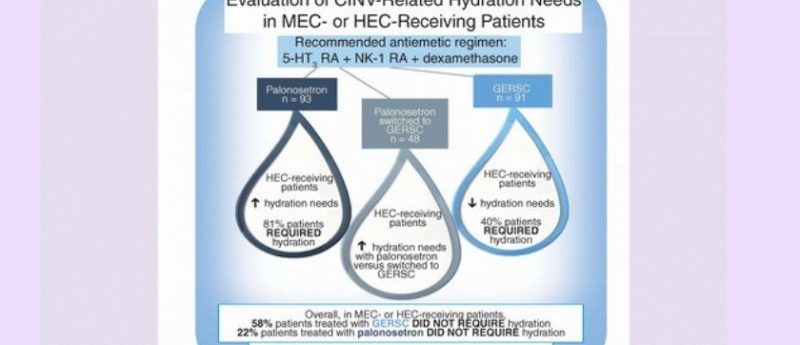
Aim: This retrospective analysis evaluated chemotherapy-induced nausea and vomiting (CINV)-related hydration needs with palonosetron or granisetron extended-release subcutaneous (GERSC), approved in 2016 for CINV prevention. Materials & methods: At a community practice, CINV-related hydration per chemotherapy cycle was determined following highly (HEC) or moderately emetogenic chemotherapy (MEC) and a guideline-recommended antiemetic regimen: neurokinin 1 receptor antagonist, dexamethasone and either palonosetron only, GERSC only, or palonosetron switched to GERSC. Results: Palonosetron-only patients (n = 93) had a significantly higher mean (standard deviation) hydration rate (0.9 [1.1]) than GERSC-only patients (n = 91) (0.3 [0.6]) (p < 0.0001). Switched patients’ (n = 48) hydration rates were significantly higher in the HEC subgroup with palonosetron (0.7 [1.2]) versus GERSC (0.5 [1.0]) (p = 0.028). Conclusion: GERSC in a three-drug antiemetic regimen may reduce hydration needs following HEC or MEC.
Poorly controlled chemotherapy-induced nausea and vomiting (CINV) negatively affects patient health, quality of life and chemotherapy compliance [1–7], and is associated with increased resource utilization and healthcare costs [7–10]. Despite comprehensive antiemetic treatment guidelines [11–14], there is still an unmet clinical need to improve the prevention of CINV.
Clinical practice guidelines recommend a combination of antiemetics of different classes for the prevention of CINV, including 5-hydroxytryptamine type 3 (5-HT3) receptor antagonists, neurokinin 1 (NK-1) receptor antagonists and dexamethasone [11–14]. Granisetron and palonosetron are two of several 5-HT3 receptor antagonist antiemetics included in antiemetic guidelines [11–14].