Turalio risk evaluation and mitigation strategy for treatment of tenosynovial giant cell tumor: framework and experience
The latest Perspective article, published in Future Oncology, discusses the use of Risk Evaluation and Mitigation Strategy (REMS) for pexidartinib in tenosynovial giant cell tumor. Read the abstracts below and read the full open access journal article here >>>
Abstract
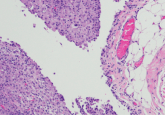
For drugs with enhanced serious safety risks, REMS may be required. Pexidartinib is approved for treatment of adult symptomatic tenosynovial giant cell tumor (TGCT) associated with severe morbidity or functional limitations and not amenable to improvement with surgery. Its approval was conditional on its prescription via a mandatory REMS due to serious and potentially fatal liver injury seen in clinical trials. Turalio® REMS aims to mitigate this risk by ensuring provider education on pexidartinib use and required REMS components, prescriber adherence to baseline and periodic monitoring, and enrolling patients in a registry to further assess safe use and acute, chronic and irreversible hepatotoxicity. Through Turalio® REMS, benefits of treating patients with pexidartinib may be preserved.
Lay abstract
For drugs with serious side effects, specific safety measures may be put in place to manage these serious side effects in the form of REMS programs. Pexidartinib (Turalio®) is approved for treatment of adults who have symptoms of severe tenosynovial giant cell tumor or have limitations in function that do not improve with surgery. Turalio® has a REMS program because liver injuries that can be serious or fatal were seen in pexidartinib clinical trials. This program aims to decrease the seriousness of the liver injuries by assuring doctors and pharmacists are educated on how to use the drug, patients are advised of this potential risk and that baseline and periodic monitoring of patients are conducted.