PIVOT-10: Phase II study of bempegaldesleukin plus nivolumab in cisplatin-ineligible advanced urothelial cancer
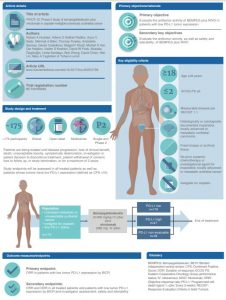
This article describes the ongoing Phase II PIVOT-10 study (NCT03785925) that is evaluating the efficacy and safety of first-line bempegaldesleukin (BEMPEG) plus nivolumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer (mUC). A total of ≈175 patients will be enrolled and treated with BEMPEG plus nivolumab, including ≈110 patients with low tumor PD-L1 expression. The study is being conducted in more than 120 sites in over 20 countries across five continents. The study participants will receive BEMPEG 0.006 mg/kg intravenously plus nivolumab 360 mg intravenously every 3 weeks and will be treated for a maximum of 2 years. The primary objective is objective response rates (ORR) per Response Evaluation Criteria In Solid Tumors version 1.1 by blinded independent central review in patients with low PD-L1 expression. The secondary objectives are ORR and duration of response in all treated patients including patients whose tumors have low PD-L1 expression, and safety and tolerability will be assessed. The pending results of the PIVOT-10 study may provide BEMPEG plus nivolumab with the potential to address an unmet need for an effective and well tolerated treatment for patients with cisplatin-ineligible advanced or mUC with low tumor PD-L1 expression.
The latest clinical trial protocol article published in Future Oncology describes the ongoing Phase II PIVOT-10 study.
Abstract
The choice of first-line therapy for patients with mUC, is based on cisplatin-eligibility and PD-L1 status. For patients with mUC who are ineligible for cisplatin and with low PD-L1 expression, chemotherapy-based regimens are the only approved first-line option. In a Phase I/II trial of the chemotherapy-free regimen, BEMPEG plus nivolumab, patients with locally advanced or mUC experienced tumor responses regardless of baseline PD-L1 expression (ORR: 50% and 45% in patients with PD-L1-positive and -negative tumors, respectively). The Phase II PIVOT-10 study, evaluates efficacy and safety of first-line BEMPEG plus nivolumab in cisplatin-ineligible patients with locally advanced or mUC. Most patients will have low PD-L1 expression. Primary endpoint: ORR (including complete response).
Lay Abstract
When people are diagnosed with advanced urothelial (bladder) cancer, they are often given a type of chemotherapy, called cisplatin, as their first treatment. However, some people are not able to receive cisplatin. For these people, especially if their cancer has a low level of the protein called PD-L1, treatment options are limited. This protein helps the body’s natural immune system to defend itself against infections and disease, including cancer. Patients with a low level of PD-L1 protein in their cancer typically have a poor outlook. This article presents information on a clinical trial called PIVOT-10. This trial will test how well as new drug that modifies the immune system, called BEMPEG combined with a drug that blocks PD-1, nivolumab, works as an initial treatment for patients with advanced urothelial cancer who cannot receive cisplatin. Most patients will have a low PD-L1 tumor expression level. The main assessment will be ORR, which measures the percentage of patients experiencing shrinkage or disappearance of their cancer after receiving treatment. PIVOT-10 will also check the safety of BEMPEG and nivolumab, and the impact of the treatment combination on survival.
View the infographic and read the full article:
Register to Oncology Central now for the latest journal content