CaboPoint: a Phase II study of cabozantinib as second-line treatment in patients with metastatic renal cell carcinoma
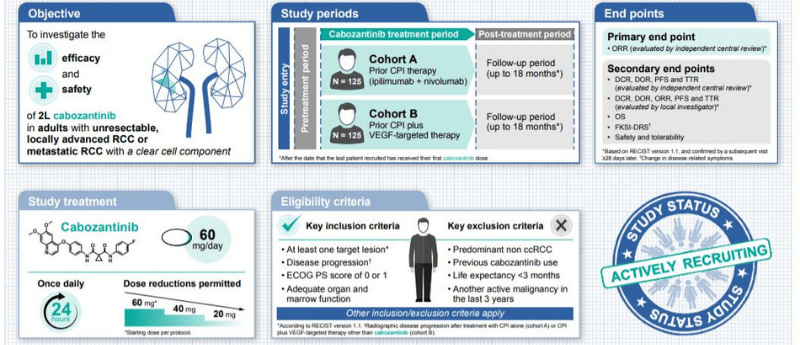
This Clinical Trial Protocol, recently published in our partner journal Future Oncology, describes the Phase II CaboPoint study (NCT03945773) that has been designed to evaluate the efficacy and safety of second-line cabozantinib in patients with unresectable, locally advanced or metastatic RCC whose disease has progressed despite 1L checkpoint inhibitor (CPI) therapy with either combination ipilimumab plus nivolumab therapy or combination CPI plus VEGF-targeted therapy other than cabozantinib. The study will enrol 250 eligible patients into two independent cohorts based on their prior therapy: prior ipilimumab plus nivolumab (cohort A, n = 125); prior CPI plus VEGF-targeted therapy (cohort B, n = 125). Patients in both cohorts will receive once-daily oral cabozantinib 60 mg. The primary endpoint is objective response rate (ORR) evaluated by independent central review. Secondary endpoints include ORR evaluated by local-investigator’s review, overall survival, progression-free survival, time to response, duration of response, disease control rate, change in disease-related symptoms, and safety/tolerability. The study is currently actively recruiting patients.
Abstract
Cabozantinib is an inhibitor of multiple tyrosine kinases, including AXL, MET, and vascular endothelial growth factor (VEGF) receptors. Here, we describe the rationale and design for the Phase II CaboPoint trial (NCT03945773), which will evaluate the efficacy and safety of cabozantinib as a second-line treatment in patients with unresectable, locally advanced or metastatic renal cell carcinoma (RCC) whose disease has progressed despite checkpoint inhibitor (CPI) therapy. Patients will be recruited into two cohorts: prior ipilimumab plus nivolumab (cohort A) or prior CPI–VEGF-targeted therapy (cohort B). All patients will receive once-daily oral cabozantinib 60 mg for up to 18 months. The primary endpoint is objective response rate. Secondary endpoints include overall survival, progression-free survival, and safety.
Lay summary
Most patients diagnosed with kidney cancer have a type of tumor termed renal cell carcinoma (RCC). The majority of cases of RCC are described as ‘clear cell’ because the tumor cells appear clear when viewed under a microscope. Cabozantinib is an oral treatment approved for use in some patients with advanced RCC, including those with clear cell disease. Cabozantinib slows RCC progression by targeting pathways that help tumors grow, including inhibition of vascular endothelial growth factor (VEGF). The ongoing CaboPoint study will assess the efficacy and safety of cabozantinib in patients with clear-cell RCC (ccRCC) that has progressed despite previous anti-cancer treatment involving an immune checkpoint inhibitor (CPI). CPI therapy helps the body to detect tumors and to launch its own anti-cancer response. Patients included in CaboPoint must be adults with ccRCC that is not suitable for surgery and has either spread within the kidney or to other organs, despite previous CPI-based therapy. In total, 250 patients will be recruited: 125 who received previous combination CPI treatment (ipilimumab plus nivolumab; Group A), and 125 who received previous CPI treatment plus anti-VEGF therapy (Group B). Patients will start cabozantinib at a dose of 60 mg/day and continue treatment for up to 18 months. The main outcome to be studied will be the number of patients with a reduction in tumor size (objective response rate). The length of time patients live with their disease, the effect of treatment on symptoms and patient safety will also be evaluated.