788 blood-based biomarkers identified for potential early detection of cancer
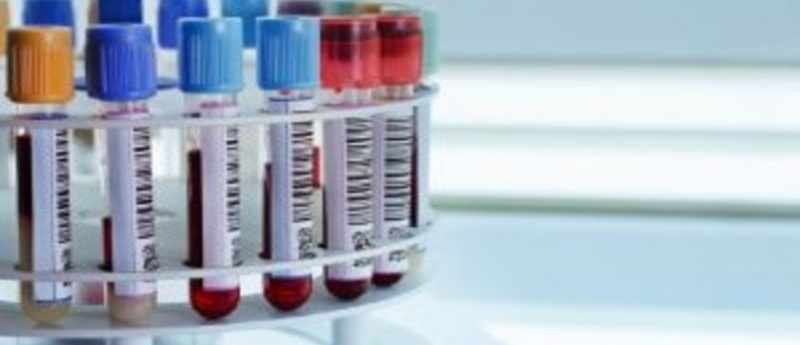
A collaborative study featuring researchers from the Universities of Sheffield, Coventry and Warwick (all UK) are the first to collate a record of relevant cancer blood biomarkers researched in the last 5 years, of which 788 have been identified. By utilizing this inventory of biomarkers, early stage cancer screening tests could be developed for the general population.
The team began with over 19,000 studies published within the last 5 years on blood-based biomarkers. After systematically reviewing the studies and ruling out those that did not match criteria – such as studies with <50 patients – they reduced the list to 4000 studies.
From these collected data the team was also able to group the biomarkers by their molecular function and record the technologies that can detect them.
Lesley Uttley, lead researcher from the University of Sheffield commented on the significance of compiling the list despite the sheer number of publications in the field:
“Our data mining approach allowed us to take in all relevant research findings from the 5 year period, which meant we could map the full range of potential blood-based biomarkers that are particularly relevant for early detection of cancer.”
The research was carried out on behalf of the Early Cancer Detection Consortium, a group consisting of nearly 40 organizations, spanning universities, hospitals and commercial companies. Funding was provided by Cancer Research UK in the hope that the investigation may lead to a cost-effective screening test to identify individuals within the general population with early stage cancers.
Further research is still required to confirm whether each biomarker is durable and could feasibly be utilized as part of a screening test. By this stage each biomarker will also be grouped by cancer type.
Subsequently the validated biomarkers will enter a clinical study, using samples from cancer patients and healthy controls, to verify how effectively they identify the presence of cancer.
Finally the successful biomarkers will undergo a clinical trial to ascertain whether the screening test is cost-effective and works in practice.
Early Cancer Detection Consortium director Ian Cree, commented “Our expectation is that, once the validation and clinical studies are completed, we will have a suite of around 50 biomarkers, identified using four different tests that can go into the clinical trial. To complete the validation and the trials will take 6–8 years, but in theory, we could have a test ready within 3 years for use in high risk groups.”
“Our vision is that the screen will pick up even the small amounts of these biomarkers that might be in the blood at an early stage of the cancer, without necessarily identifying which cancer they relate to. Patients would then be referred for more specific tests, that could narrow down the tumor type.”
Pinpoint Cancer Ltd, is a company established by the Consortium to take this research forward and is actively seeking investment and funding for next stage of the project.
Sources: Uttley L, Whiteman BL, Woods HB, Harnan S, Philips ST, Cree IA.Building the Evidence Base of Blood-Based Biomarkers for Early Detection of Cancer: A Rapid Systematic Mapping Review, EBioMedicine, doi: 10.1016/j.ebiom.2016.07.004, (2016) (Epub ahead of print);
The University of Sheffield press release