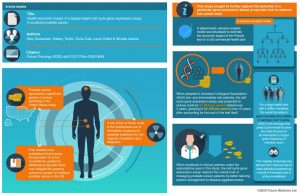
The health economic impact of a biopsy-based cell cycle gene expression assay in localized prostate cancer: a peek behind the paper with Gary Gustavsen

Take a peek behind the paper with Oncology Central as we speak with Gary Gustavsen (Health Advances, MA, USA) about his recently published paper in Future Oncology discussing the health economic impact of a biopsy-based cell cycle gene expression assay in localized prostate cancer.
Please introduce yourself, your research background and current research interests.
My name is Gary Gustavsen and I am a Partner at Health Advances, a healthcare-focused strategy consulting firm. My research interests focus heavily on the emerging field of precision medicine. We work with both diagnostic and pharmaceutical companies to identify optimal approaches to develop and commercialize novel diagnostic advances. Many of these diagnostics deliver significant medical value yet struggle to gain early acceptance due to the required shifts in patient management.
What are the current challenges for prostate cancer in the US and how will this continue to evolve?
Prostate cancer is a unique tumor type in that many men will die ‘with prostate cancer’ rather than ‘of prostate cancer’. Prostate cancer is incredibly heterogeneous, with some tumors being very indolent and slow growing. PSA screening has allowed us to identify these tumors with great efficiency, yet many men will die of some other cause before their prostate cancer becomes clinically significant. For these men, the morbidity of surgery or radiation therapy represents an unnecessary risk to their health.
Please can you give an overview of your recent article published in Future Oncology?
This study aims to assess a precision medicine test designed to address this key challenge. By genomically classifying tumors we can better define the risk of patient mortality, and therefore better tailor the aggressiveness of treatment. This test has previously been clinically validated, but this study investigates the economic impact of the assay, in order to help insurance companies make more informed coverage decisions and medical policy.
How do you think the results can help to overcome the future challenges with prostate cancer surveillance?
Previous studies have shown that incorporation of this test into clinical practice will reduce unnecessary surgery and radiation therapy. This study demonstrates that adoption of the test carries a significant economic impact as well. This data should help reduce reimbursement barriers to the test and thus reduce unnecessary treatment in real world clinical practice.
Where do you see prostate cancer surveillance field in 5 years’ time?
With the broader adoption of precision medicine tools in prostate cancer, over-treatment will be significantly reduced, with a substantial proportion of men opting for “active surveillance” rather than immediate treatment.
View the infographic and read the full article:
The opinions expressed in this interview are those of the interviewee and do not necessarily reflect the views of Oncology Central or Future Science Group.
Register to Oncology Central now for the latest journal content

Gary Gustavsen joined Health Advances in 2005 and leads the firm’s Precision Medicine Practice. A noted writer and workshop leader in the field of companion diagnostics and precision medicine, his work focuses on commercialization strategy, indication prioritization, pricing and reimbursement strategy, system economics, and business development opportunities for both diagnostic and therapeutic clients.
Prior to joining Health Advances, Gary was a researcher at Brookhaven National Lab evaluating a proprietary line of synthetic growth factors. Gary also worked in the Cell & Tissue Technologies group at Becton Dickinson, the Exploratory Cancer Research group at OSI Pharmaceuticals, and most recently the Corporate Strategy group at Millennium Pharmaceuticals. Gary received his BSE degree in Biomedical Engineering from Duke University and his MS degree in Biomedical Engineering from Stony Brook University.