Phase I dose-escalation study of NKTR-255 (IL-15 agonist) for natural killer cell immunomodulation in hematologic malignancies
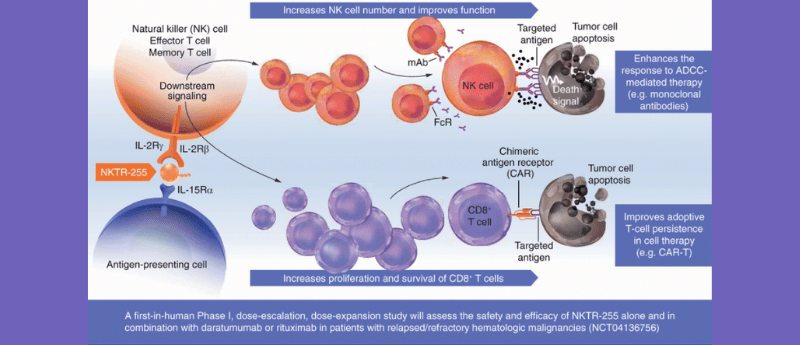
Recently, Future Oncology published the protocol and rationale for an ongoing Phase I dose-escalation study of an investigational interleukin-15 agonist – NKTR-255 – for use in multiple myeloma or non-Hodgkin’s lymphoma. The trial is building on in vivo research demonstrating its immunomodulatory effects on natural killer (NK) cells; enhancing NK cell activity has been shown to have anti-tumor effects in hematologic malignancies. The Phase I study is investigating NKTR-255 as monotherapy and in combination with either daratumumab (Darzalex®; CD38-targeted monoclonal antibody) or rituximab (Mabthera®; CD20-targeted monoclonal antibody). The trial will recruit patients with relapsed/refractory multiple myeloma, or non-Hodgkin’s lymphoma with documented disease progression.
Abstract:
NKTR-255 is an investigational polyethylene glycol-modified recombinant human IL-15 (rIL-15) receptor agonist, designed to improve the immunotherapeutic and anti-cancer benefit observed with rhIL-15 while circumventing the toxicities associated with this therapy. In preclinical studies, NKTR-255 has demonstrated enhanced proliferation and function of CD8+ T cells and natural killer cells, as well as enhanced anti-tumor activity and survival both as monotherapy and in combination with monoclonal antibodies in multiple cancer models. Here, we describe the rationale and design of the first-in-human Phase 1, dose-escalation and dose-expansion study of NKTR-255 alone and in combination with daratumumab or rituximab in adults with relapsed/refractory multiple myeloma or non-Hodgkin’s lymphoma (NCT04136756) that will determine the maximum tolerated dose and recommended Phase 2 dose for NKTR-255.
Lay Abstract:
Interleukin-15 (IL-15) is a protein that helps the body’s natural immune system to defend itself against infections and diseases like cancer. This article discusses a clinical trial in patients with multiple myeloma or non-Hodgkin’s lymphoma that evaluates a new investigational medicine, NKTR-255, a polymer-modified form of IL-15 that has been engineered to improve its ability to provide a sustained anti-tumor immune response. The trial will explore different doses of NKTR-255 to determine patient side-effects and to find the highest acceptable dose that patients can tolerate. Based on this, a dose will be chosen that offers an optimal balance between having a positive anti-cancer effect and minimizing side-effects. This dose will be tested further in patients who have had different treatments in the past. If the side-effects are acceptable, this dose will be tested in a new trial in a large number of patients.
Read the full paper ‘Phase I dose-escalation study of NKTR-255 (IL-15 agonist) for natural killer cell immunomodulation in hematologic malignancies‘: